At the recent 2021 PDA Pharmaceutical Microbiology Conference, Ken Boone from Merck presented on the recent article he had co-authored with Ed Tidswel in the PDA Journal about detecting Cutibacterium acnes (C. acnes). In it, he discusses the issue that C. acnes is a relatively common product contaminant, but traditional environmental monitoring methods generally fail to detect its presence. Click here to read full abstract.
Skin C. acnes — One of the Most Prevalent Contaminants
It’s well recognized that people are the major risk for microbial contamination in pharmaceutical cleanrooms. Based on the Human Microbiome Project, C. acnes is one of the most prevalent microorganisms found on skin. Considering this, one would expect that C. acnes would be detected at levels that are at least similar to those of other skin microorganisms however, this is not the case.
How Traditional Environmental Monitoring Fails
The reasons for this lack of recovery are the incubation conditions and media selected for use in most environmental monitoring programs. While aerotolerant, C. acnes prefer anaerobic conditions and therefore will not grow when incubated aerobically. It also grows best when a complex growth media is used. Without the desired growth factors, any growth will be too slow to allow for detectable colonies to form within the incubation periods most often used for environmental monitoring samples. By comparison, the media and incubation scheme utilized for sterility testing are capable of recovering C. acnes. In fact, there have been numerous 483s citing that sterility test failures caused by C. acnes were indicative of inadequate controls to prevent contamination from personnel.
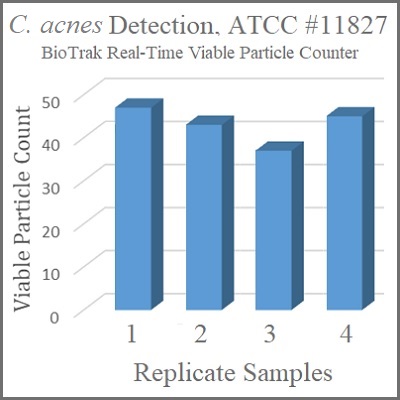 Use Real-Time Detection to Mitigate Risk
Use Real-Time Detection to Mitigate Risk
There is one obvious benefit to being able to reliably detect the presence of C. acnes prior to the sterility test—it allows for actions to be taken to prevent contamination rather than detecting it in the final product. Making the required changes to the traditional culturing methods to allow for detection would make them very burdensome and costly to perform. Fortunately, there is an alternative—the TSI BioTrak® Real-Time Viable Particle Counter. Being a biofluorescent particle counter, it does not rely on growth for detection, but instead detects the intrinsic fluorescence of microbial cells.
Proof in Testing
During validation testing of the BioTrak Real-Time Viable Particle Counter, the ability to detect a panel of microorganisms was assessed—including C. acnes. This testing confirmed the capabilities of the instrument to detect this important, but currently under-detected, viable contaminant. Not only is it capable of detection, it also provides results in real-time. This allows for immediate actions to be taken to mitigate the risk to product – keeping patients safe.
 Français
Français
 汉语
汉语
 English
English
 Français
Français
 Deutsch
Deutsch

