Many facilities choose to install facility monitoring systems to monitor particle counts and other environmental parameters. Why not, as there are multiple benefits in use, and it makes great business sense. However, benefits to a business are not always fully understood. They include:
- Reduced waste
- Improved yield
- Improved quality
- Increased profits
While there are some that choose to install a facility monitoring system just because regulatory guidance states one should be installed and used, many, given the choice, would choose not to. Initial capital and ongoing maintenance costs seem expensive. The mountains of data that will require analysis seems daunting, as do alert and action level excursions leading to time consuming root cause investigations. Plus, consideration for the maintenance, calibration and validation overhead involved.
Question — Why do Regulations Expect a Monitoring System to Be Installed?
Answer — Risk Reduction, and it makes great business sense.
A facility monitoring system improves probability of hazard detection, leading to a reduction in risk. Product quality is impacted if too many airborne particles find their way into sterile products, compromising patient safety. Only when deploying and correctly positioning monitoring probes to frequently collect data, is there a chance of detecting particles. If there are no particle monitoring probes installed close to critical processing locations, the probability of detecting particles entering the process is zero.
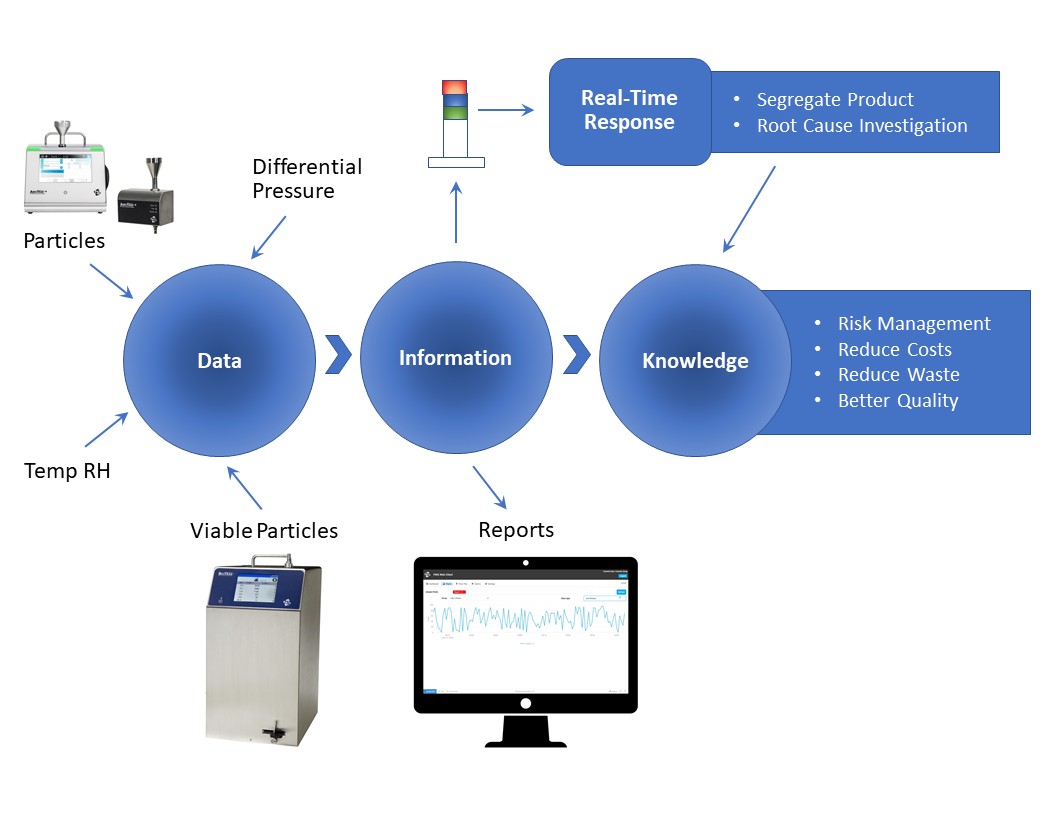
Turning critical data into information is key—real-time data presentation, reports, and alarm notifications. This results in increased knowledge and a better understanding of the manufacturing process. Increased knowledge leads to recognizing when the process is drifting out of control before it’s too late. This means less segregated product, less product waste and fewer interruptions during manufacturing—without compromising patient safety.
The purpose of a facility monitoring system― Data to Information to Knowledge.
Monitoring Makes Great Business Sense
Today, monitoring systems are already being used to support energy saving initiatives. There are significant energy savings to be made when setting back air change rates and air velocities—being safe in the knowledge that environmental conditions have not been compromised. Continuous particle monitoring in a facility means the exact time of a particle excursion is known and immediately notified to end users. This supports timely root cause investigations and minimizes how much of the batch is segregated—saving a significant amount of money.
The availability of Alternative Microbiological Methods (AMMs), such as biofluorescent particle counters (BFPC), means there is also an option for immediate understanding of the microbiological quality of the air surrounding the process. This can provide real-time process control with no delay or risk to process from interventions.
Smart factories of the future will have fully interoperable systems where data is seamlessly exchanged between multiple platforms. Sharing and centralizing facility monitoring system data transforms it into holistic information that aids decision making. This holistic information could predict that an excursion is likely, enabling proactive steps to be taken to positively impact yield and significantly save on manufacturing costs.
Facility Monitoring Makes Sense - An Everyday Example
Think of a facility monitoring system like a home refrigerator or freezer. They efficiently monitor their internal temperature to ensure preservation of items stored inside.
Food stored at an incorrect temperature is potentially dangerous to human health. It is likely to perish and will need to be thrown away. Food that is supposed to simply be kept cool might get frozen, becoming inedible and go to waste. Energy is wasted.
Continuously monitoring the internal temperature of a refrigerator or freezer reduces the risk of food spoiling and protects people from getting food poisoning. Efficient temperature monitoring saves energy, maintains food quality and reduces waste while saving money.
In the same way, it makes sense to continuously monitor the environment surrounding a manufacturing process when it’s critical to product quality.
Click here for more information about TSI Facility Monitoring Software.
 汉语
汉语
 English
English
 Français
Français
 Deutsch
Deutsch